C2h4 Hybridization
In the ethene molecule the carbon atoms are sp2 hybridized. The chemical formula C2H4 represents Ethylene.

Hybrid Orbitals In Carbon Compounds Chemistry Classroom Teaching Chemistry Organic Chemistry
The first and foremost thing that we need to look into while finding out the hybridization of any molecule is the electronic configuration of the atoms.

C2h4 hybridization. Ethene C2H4 has a double bond between the carbons. This is similar to sp3 hybridisation except there are only 2 hydrogen nuclei pulling on the bonding electrons which need an electron each and the other 2 electrons are required for the π pi bond double bond between the two Carbons. Hybridization is the process of mixing two or more atomic orbitals to create new covalently bonded orbitals in molecules.
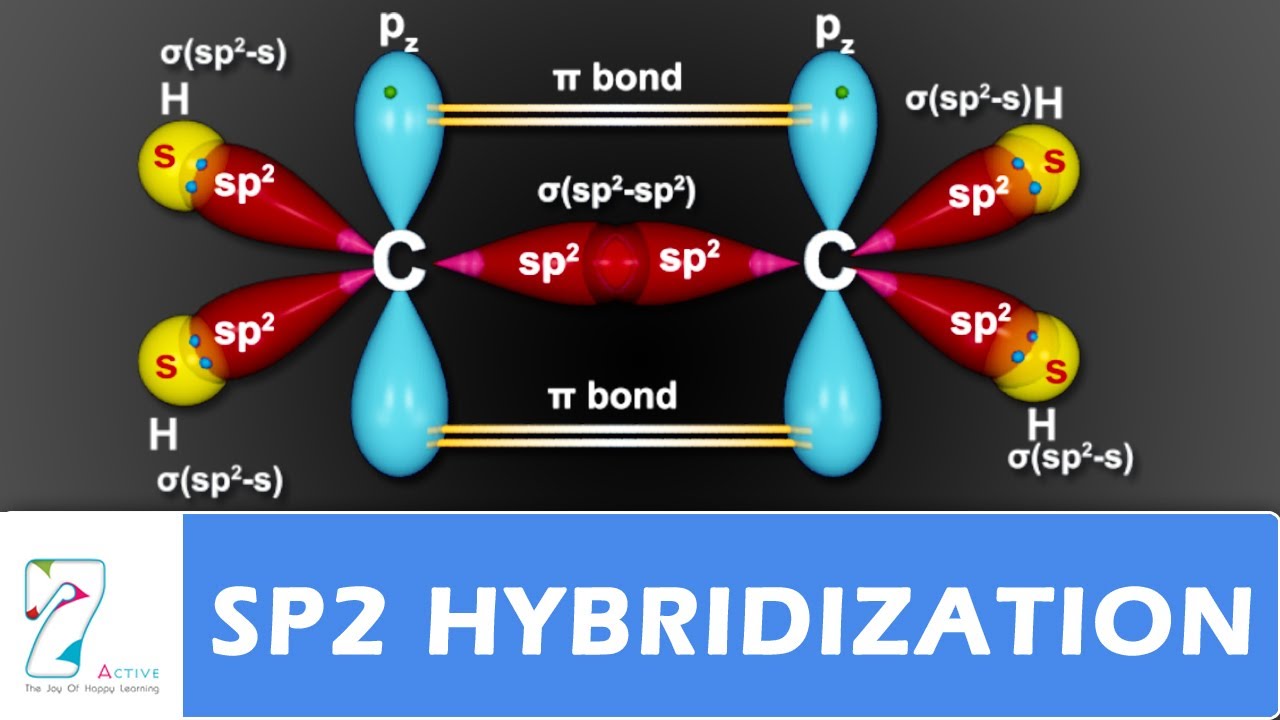
This molecule is also represented by H2CCH2 clearly showing the alkene nature of the compound. Atomic orbitals combine together to form hybrid orbitals and the process is known as hybridization. These overlap above and below the C-C sigma bond to make a two lobed pi bond.
Hybridisation In the case of ethene there is a difference from say methane or ethane because each carbon is only joining to three other atoms rather than four. July 30 2019 C 2 H 4 Hybridization In the formation of ethene molecule one of the sp 2 hybrid orbitals of carbon atom overlaps axially with sp 2 hybridized orbitals of another carbon atom to form C-C sigma bond. C2H4 exists as a colorless gas and is flammable.
Discuss the bonding in C 2 H 4 in terms of a suitable hybridization scheme. Why C 2 H 4 forms Pi bonds in its hybridization scheme. While the other two sp2 hybrid orbitals of each carbon atom are used for making sp2-s sigma bond with two hydrogen atoms.
In the formation of ethene molecule one of the sp2 hybrid orbitals of carbon atom overlaps axially with sp2 hybridized orbitals of another carbon atom to form C-C sigma bond. Possible question that can be asked in the test. The type of hybridization involved with CH4 is sp 3.
We will discuss in detail how this hybridization occurs below. Therefore hybridization of C2H4 is Sp². According to the C2H4 lewis dot structure carbon is the central atom and each carbon is attached to three atoms 1 Carbon 2 Hydrogen.
In ethylene each carbon combines with three other atoms rather than four. In this Hybridization one s and two p orbitals are mixed to give three new sp2 hybrid orbitals which all are in the same shape and equivalent energies. When the carbon atoms hybridise their outer orbitals before forming bonds this time they only hybridise three of the orbitals rather than all four.
These three sp2 hybrid orbitals are at. Each carbon in the Ethene Lewis structure contains Sp². In C2H4 there are 3 bonds around C - 3 x sigma bonds and you only need an s and two p orbitals to do this - sp2 hybridisation.
According to the c2h4 lewis dot structure carbon is the central atom and each carbon is attached to three atoms 1 Carbon 2 Hydrogen. An alkene is a hydrocarbon with a Carbon-Carbon double bond. In the molecule ethene both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital.
Ethene C 2 H 4. In order to understand the hybridization of CH 4 methane we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial.
Sp2 Hybridisation in Ethene C2H4. Geometry and hybridization approach of e. The two carbon atoms form a sigma bond in the molecule by overlapping two sp2 orbitals.
However hybrid orbitals and pure atomic orbitals have different molecular. C2H4 Lewis Structure Molecular Structure Hybridization Bond Angle and Shape. Find the type of hybridization in C 2 H 4 omlecule.
The correct Lewis structure for ethene is shown below. Also lone pair present on the carbon is zero. Type of hybridization in C2H4.
Shields demonstrates with an example how to draw the sigma bonding system and the pi bonding in ethene ethylene. Ethene is the simplest alkene compound in alkene compound series. Describe the type of bonds present in C 2 H 4 using hybridization scheme.
First of all write orbital diagrams for Carbon and. Also lone pair present on the carbon is zero. This video gives information about orbital hybridization specially sp2 and sp hybridization and shapes of molecules.
The remaining electron on each C atom is a two lobe p orbital. Name of the Molecule. Hybridization of Ethene C2H4 C2H4 has an sp2 Hybridization process.
One unpaired electron in the p orbital remains unchanged. Hybridization of atoms in ethene molecue can be found from lewis structure. C2H4 n 42 74 8 28 36 nn4 368 4 4 Lone pair 42 2 N 4 2 6 sp3d2.
So H 3 0 3 is the hybridization number for C2H4. So H 3 0 3 is the hybridization number for C2H4. Each carbon in the C2H4 Lewis structure contains Sp².
These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped probability areas above and below the plane of the six atoms. Orbital hybridization is discussed. Therefore hybridization of C2H4 is Sp².
In a double bond we have one sigma and one pi bond.

How To Write The Name For Mg Hco3 2 In 2021 Names Writing Molecules

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Lewis Molecules Chemical Formula

Hybridization Of C2h4 Molecular Geometry Electron Configuration Pi Bond

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Lewis Molecules Chemical Formula

Bro3 Lewis Structure Molecular Geometry Hybridization Polar Or Nonpolar In 2021 Molecular Geometry Molecular Shapes Molecular

Pin Pa 100 Steps To Sat Ii Chemistry

Becl2 Lewis Structure Beryllium Chloride In 2021 Lewis Molecules Chemical Formula

Ethylene Sp2 Hybridization Mario Characters Illustration Gaming Logos

Chlorine Trifluoride Clf3 Lewis Structure Molecular Geometry Polar Or Non Polar In 2021 Molecular Geometry Molecular Shapes Molecular

Nitrogen Trifluoride Nf3 Lewis Structure Polar Or Non Polar Molecular Geometry Shape In 2021 Molecular Geometry Molecular Shapes Molecular

Ethene Reactions Of Ethene Properties Of Ethene Chemistry Education Teaching Chemistry Chemistry Help

Atomic Orbital Hybridization Sp3 Mr Causey S Chemistry Youtube Chemistry Basics Organic Chemistry Organic Chem
Atomic Orbital Hybridization Sp3 Mr Causey S Chemistry Youtube Chemistry Basics Organic Chemistry Organic Chem

Molecular Orbitals Of H2 Chemistry Libretexts Chemistry Textbook Chemistry Jobs Electron Configuration

What Is The Difference Between 1s And 2s Orbital Updated Different Push Pin

Pin On Chemistry Section

Hybrid Orbitals Explained Valence Bond Theory Crash Chemistry Academy Chemistry Chemistry Help Molecular Geometry

Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride In 2021 Molecular Geometry Molecular Molecules

Hybridization Of Sf6 Sulfur Hexafluoride In 2021 Molecules Chemical Formula Sulphur